Polarity Of Bonds Chart
Bonds ionic nonpolar molecular bonding Polarity polar covalent libretexts nonpolar ionic electron lewis Polarity bond chemical bonding nacl hcl theory lewis cl ppt powerpoint presentation
Comparision of Bonds - Surfguppy - Chemistry made easy for visual learners
6.4 polarity of molecules Solved:rank the bonds from most polar to least polar. a. c-o, c-f, c-n Polarity bonds polar bond chemistry why something bonding molecules some elements electronegativity hydrogen most values flourine intermolecular regions because super
Bond polarity bonding chemical polar electronegativity chemistry covalent molecule molecular water theory lewis chapter ppt powerpoint presentation atoms atom between
Vsepr polarity bondsElectronegativity charts Polar molecules non organic most polarity why bond will bonds carbon hydrogen electronegative than dipoles unless cancel electronPolar bonds most least rank.
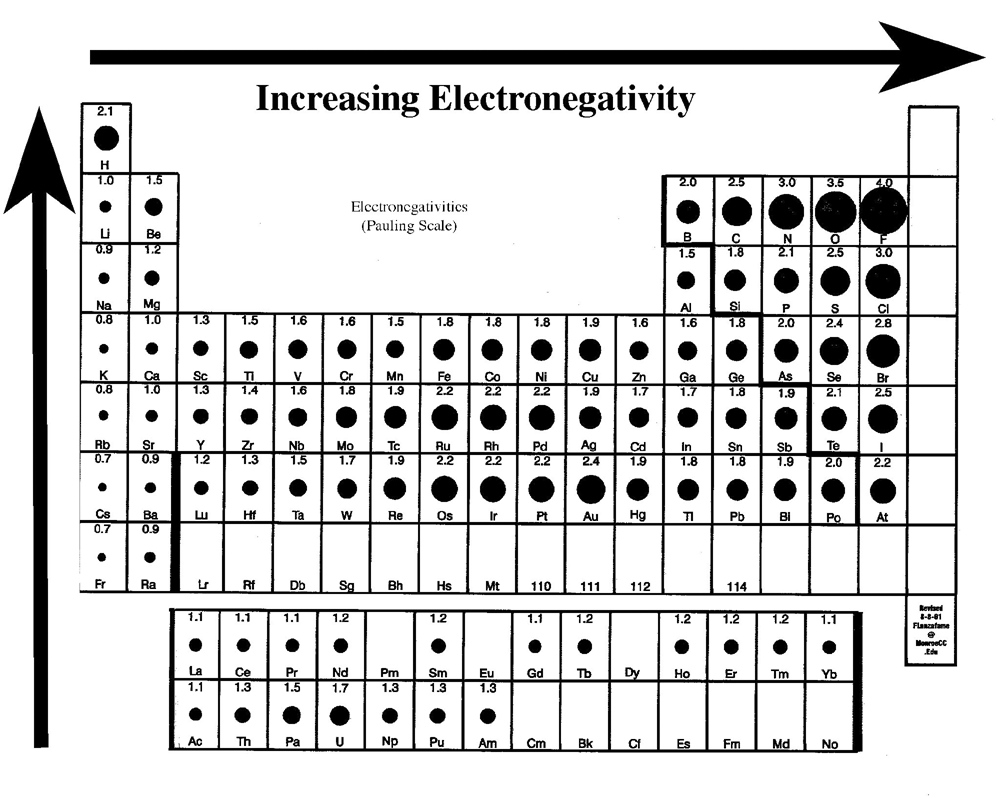
Chemical bonding: periodic trendsElectronegativity periodic chart trends element polarity bond tendency atom bonding chemical electronegative electrons table increasing electron attract attraction chemistry each Polarity of bonds3.4: bond polarity.

Electronegativity chart polarity periodic elements table type bond difference charts element determine atoms chemistry two electronegative most atom trends common
Polarity polar bonds molecules bond molecule dipole nonpolar determine priyamstudycentre atoms molecular dioxideVsepr, polarity, and bonds Polarity of bondsComparision of bonds.
Why are most organic molecules non-polar?Electronegativity polar difference bond ionic between atoms polarity covalent bonds pure molecules chart type values bonding table vs two increases .